Global regulation of drugs, medical devices, and related pharmaceutical products shares a common vision: to drive innovation while protecting public health. With the rapid advancement of science and technology, numerous innovations in medical technologies related to pharmaceutical products are emerging, presenting significant challenges to regulatory practices. Regulatory science is a relatively new scientific discipline that aims to address the needs of drug regulation by developing new tools, standards, and approaches through cutting-edge scientific research to aid decision-making in assessing the safety, efficacy, quality, and performance of regulated products. It also fosters scientific exchange and innovation through the collaborative participation and effective interaction of key stakeholders in the pharmaceutical system.
The Centre for Pharmaceutical Regulatory Sciences (CPRS) at the University of Macau, established in 2023, represents a pioneering initiative in the region. As the first center of its kind, CPRS is dedicated to driving innovation, promoting the translation of research into practical applications, and protecting public health. This commitment is reflected in its focus on advancing the discipline of pharmaceutical regulatory science through cutting-edge research, comprehensive education, specialized training, and robust collaboration.
CPRS serves as a vital hub for communication and collaboration within the Guangdong-Hong Kong-Macao Greater Bay Area. By fostering regulatory capacity building, striving for regulatory excellence, and promoting regulatory convergence, CPRS aims to enhance the overall regulatory landscape. The center brings together a diverse group of renowned experts who contribute their scientific expertise to the evaluation of various pharmaceutical products. These products include innovative medicines, Traditional Chinese Medicine products, and medical devices, ensuring a broad scope of regulatory oversight and innovation.
Leveraging its international attributes and historical connections, CPRS is envisioned to play an integral role in strengthening pharmaceutical systems. This role is not limited to the local region but extends to neighboring areas, Portuguese-speaking countries, and other international counterparts. By fostering scientific exchange and innovation, CPRS aims to drive the development of the pharmaceutical industry on a global scale.
The center’s strategic location and collaborative approach enable it to act as a bridge between different regulatory environments, facilitating the sharing of best practices and the harmonization of standards. This, in turn, supports the development of more effective and efficient regulatory frameworks that can adapt to the rapidly evolving landscape of pharmaceutical sciences.
In summary, CPRS at the University of Macau is poised to make significant contributions to the field of pharmaceutical regulatory science. Through its dedication to research, education, and collaboration, it aims to drive innovation, protect public health, and foster a global network of regulatory excellence. This vision positions CPRS as a key player in the ongoing efforts to enhance the safety, efficacy, and quality of pharmaceutical products worldwide.
Vision
Our vision is to play a pivotal role in advancing and adopting regulatory science to strengthen pharmaceutical systems, foster innovation and regulatory excellence, protect public health, and drive the development and internationalization of the health industry.
Mission
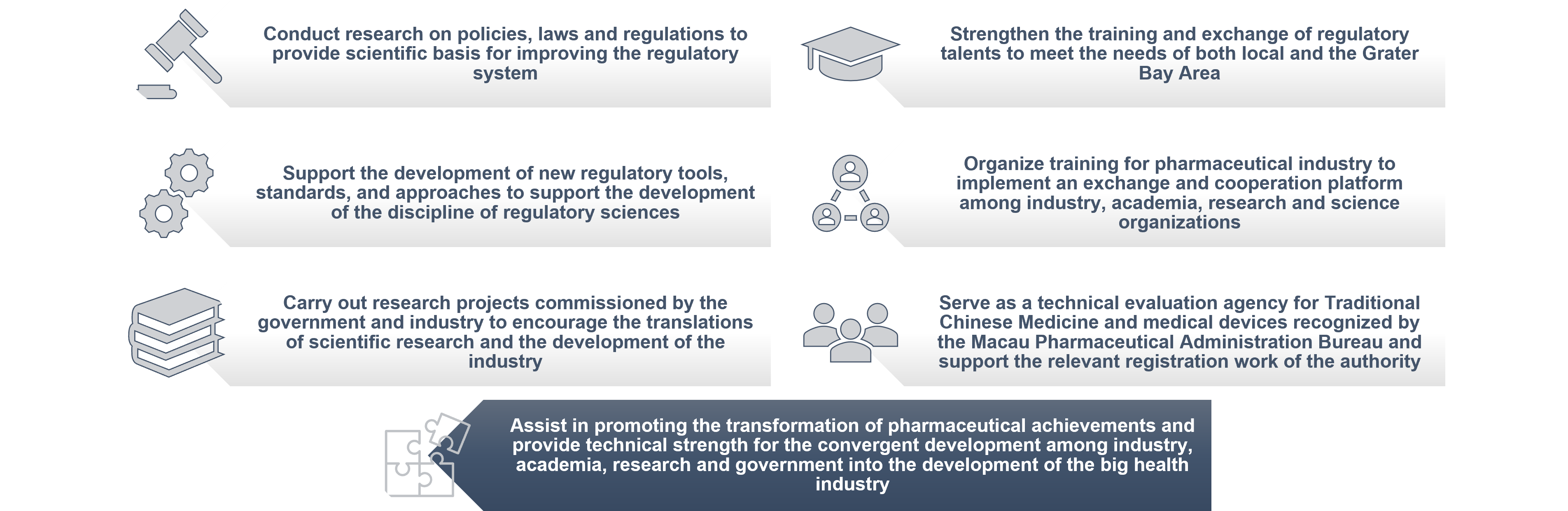
The mission of the Centre for Pharmaceutical Regulatory Science is to advance pharmaceutical regulation through innovative research, comprehensive education, and strategic collaboration. As a hub for communication and collaboration, we aim to build regulatory capacity, achieve regulatory excellence, and promote regulatory convergence in the Guangdong-Hong Kong-Macao Greater Bay Area and beyond. Locally, we strive to enhance public health and pharmaceutical innovation by ensuring robust evaluation of the safety, efficacy, and quality of pharmaceutical products including innovative medicines, Traditional Chinese Medicine products, and medical devices.